Biocognon’s Nanobody discovery platform is deeply rooted in Fluorogen Activating Protein (FAP) technology developed over the past two decades at Carnegie Mellon University. FAPs offer powerful advantages over current fluorescence based cell sorting or imaging methods used for screening antibodies. CMU researchers and collaborators have published over 150 papers developing FAP technology for diverse biological applications. Sharp Therapeutics and SpectraGenetics are Pittsburgh CMU spin-offs based on variants of FAP technology. See our Research page for a list of publications and illustrative examples.
Crucially, as described on Biocognon’s Mission page, FAP-based biosensors enable the development of a yeast secrete-and-display Nanobody discovery platform where multiple co-expressed target antigens can be combinatorially screened (ref. 152). A transformative capability of Biocognon’s platform is efficient conversion of genetic information into screenable antigens without the need of purifying those antigens one-by-one. Our FAP-based biosensors facilitate the far-reaching application of synthetic biology and supervised machine learning to antibody discovery.
While details of the FAP-based biosensors used in our Nanobody discovery platform are unpublished, it is helpful to understand the basic principles that make the FAP technology so useful. FAPs are ~30 kilodalton single chain antibody fragments that specifically and tightly bind small non-fluorescent dyes (fluorogens). These fluorogens become intensely fluorescent only when bound to a cognate FAP. One can selectively fluorescently label proteins expressed at the surface of a cell by directly adding cell impermeant derivatives of fluorogens. No washes to remove unbound (non-fluorescent) fluorogen are necessary.
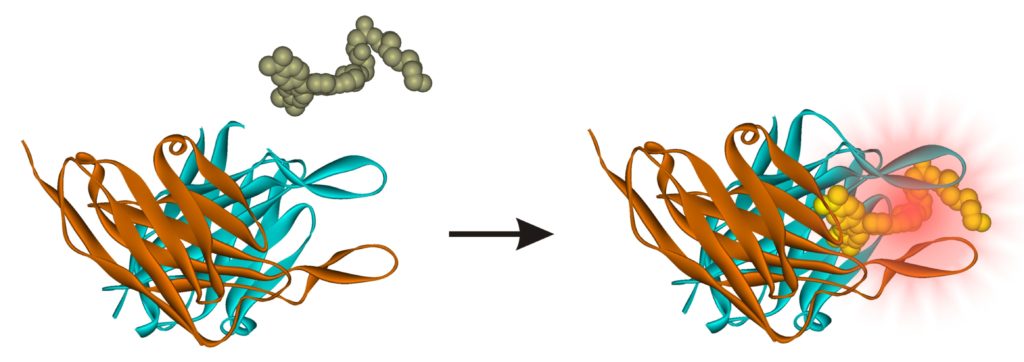
This efficient and inexpensive labeling method is ideal for developing a yeast co-expressed secrete-and-display screening platform because FAPs fused to surface-displayed Nanobodies and captured cognate antigens each fluoresce in different colors, and thus directly facilitate quantitative ratiometric fluorescence activated cell sorting. Current fluorescent labeling methods, principally using anti-FAP antibodies chemically conjugated to a fluorescent dye or genetically fused to an enzymatic fluorescent reporter, would be far more expensive and cumbersome. Fluorescent proteins cannot be used because they generate interfering fluorescent signals from inside the cells.
The simplicity and power of FAPs make them very useful tools for antibody discovery and cell and animal research, but FAPs can also be incorporated into actual products. FAPs may be used as a cheap instant method to non-covalently label antibody-FAP fusion proteins for diagnostic platforms. Our fluorogens include PEG linkers that may be coupled to pharmaceutical payloads such as toxins or radionuclides. Antibody-FAP drug conjugates (ADCs) may be non-covalently created onsite using a simple procedure that fluorescently reports stoichiometric self-assembly of the complex. This alternative mode of building ADCs may be especially important in the case of extremely labile and expensive radionuclides.
The ‘plug-in’ architecture, where payloads or sensor components are conjugated to fluorogens, and then delivered at will to genetically encoded FAP-fusion proteins in cells and animals, enables the construction of a wide variety of reporters and sensors for diverse biological applications. At will delivery of membrane permeant and impermeant fluorogens supports fluorescent reporting in time and space, facilitating kinetic studies of protein transport in cells and in the blood and tissues of animals.
‘Plug-in’ Nanobody-FAP or antibody-FAP fusions greatly extend the usefulness of Nanobodies for biomedical research and therapeutic applications because they don’t rely on genetically encoded host proteins fused to FAPs. This is a driving motivation for our Nanobody discovery platform.